La AEMPS informa sobre datos clínicos observados con el uso de rucaparib (▽ Rubraca®) como tratamiento oncológico en tercera línea o posterior | Agencia Española de Medicamentos y Productos Sanitarios
Clovis Oncology, Inc. - Clovis Oncology Announces Availability of Rubraca®▽ (rucaparib) Tablets for Women with Relapsed Ovarian Cancer in Germany

Cancers | Free Full-Text | The Development of Rucaparib/Rubraca®: A Story of the Synergy Between Science and Serendipity
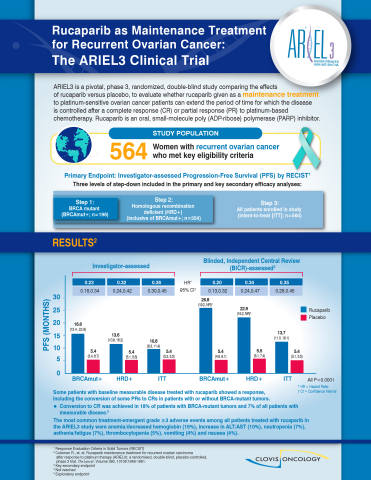
Clovis Oncology Receives EMA Validation for its Application for a New Indication for Rubraca®▽ (rucaparib) as Maintenance Tre
Rucaparib (Rubraca®): interim data from Study CO-338-043 (ARIEL4) show a decrease in overall survival compared to standard of c

CHMP recommends four new medicines, including first-in-class treatment for follicular lymphoma | RAPS