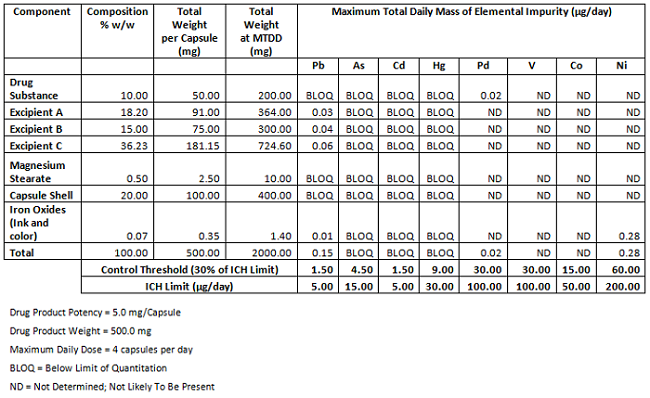
Mandates for Elemental Impurities FDA (USP) <232> & <233> / EMA (EP) Ch. 5.2 / & ICH-Q3D Step 4 version

Elemental Impurities Tests for Pharmaceutical Products According to the New ICH Q3D and USP 232/233 Guidelines - Life Science Training Institute

USP 232 and 233: Understanding Method Requirements and Guidance for Laboratory Implementation - YouTube

Metal Impurities in Pharmaceuticals and Dietary Supplements: Implementing ICP-MS for USP and Proposition 65 | American Laboratory
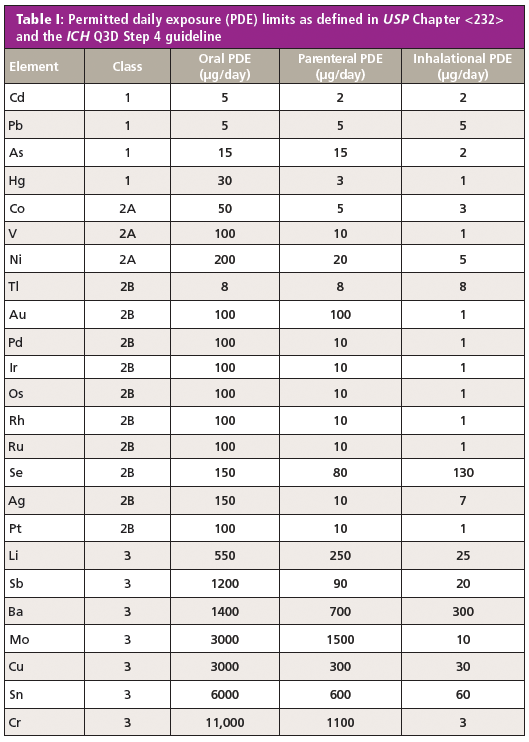
From Heavy Metals Testing to the Measurement of Elemental Impurities in Pharmaceuticals: Over 100 Years in Making the Change
Mandates for Elemental Impurities FDA (USP) <232> & <233> / EMA (EP) Ch. 5.2 / & ICH-Q3D Step 4 version