Guideline on equivalence studies for the demonstration of therapeutic equivalence for locally applied, locally acting products i

Overview of the European Medicines Agency's Development of Product‐Specific Bioequivalence Guidelines - Sullivan - 2018 - Clinical Pharmacology & Therapeutics - Wiley Online Library
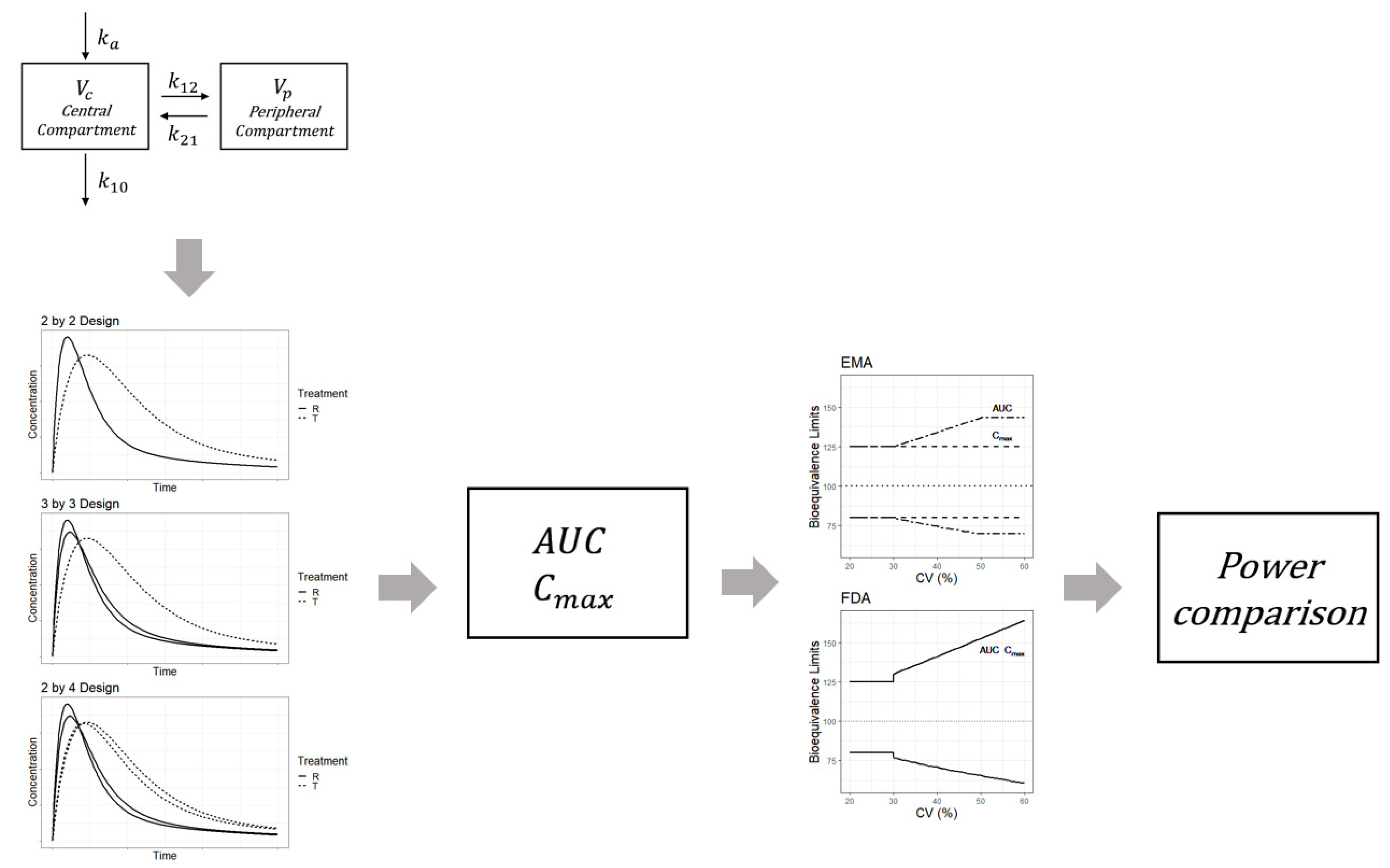
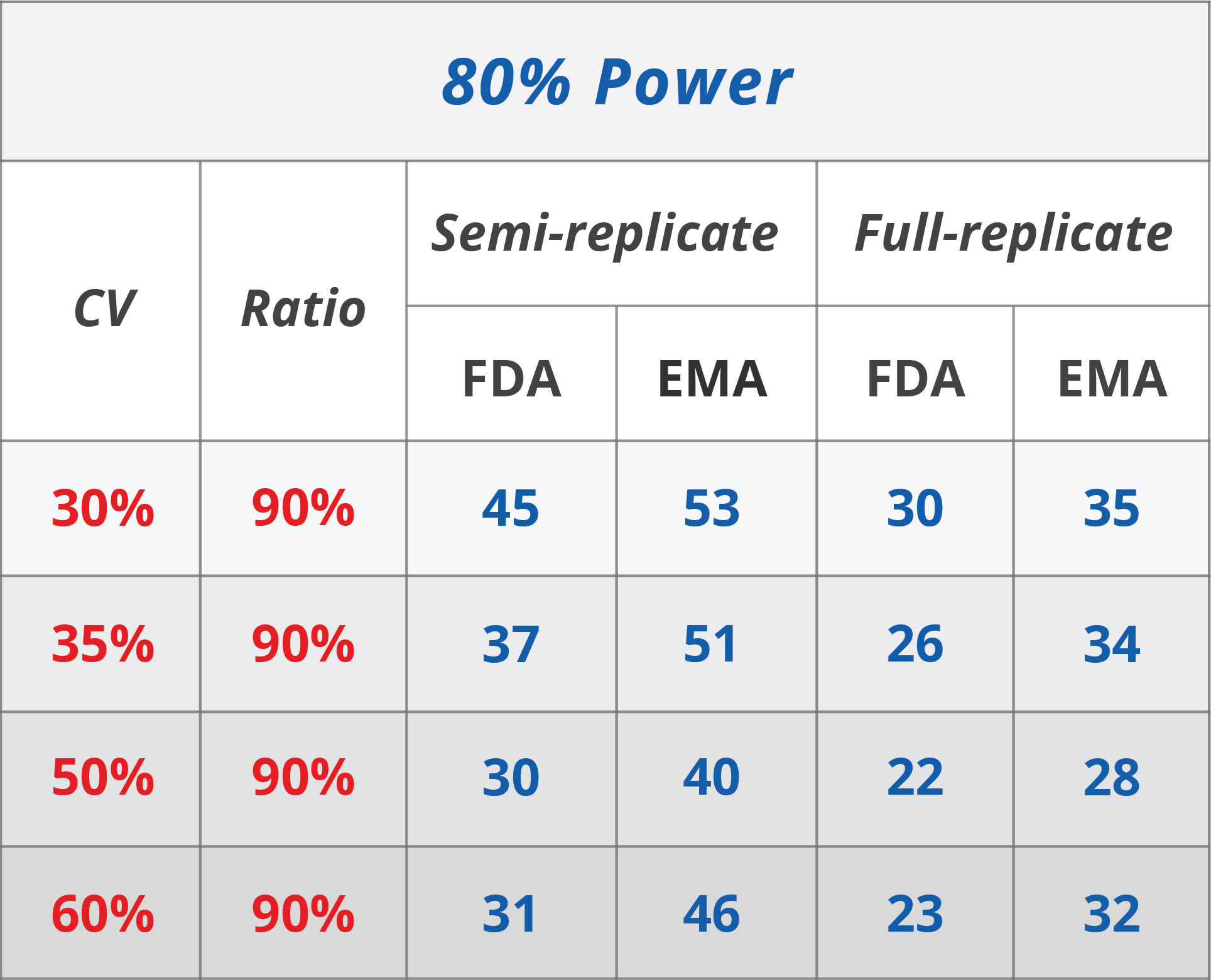
Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs
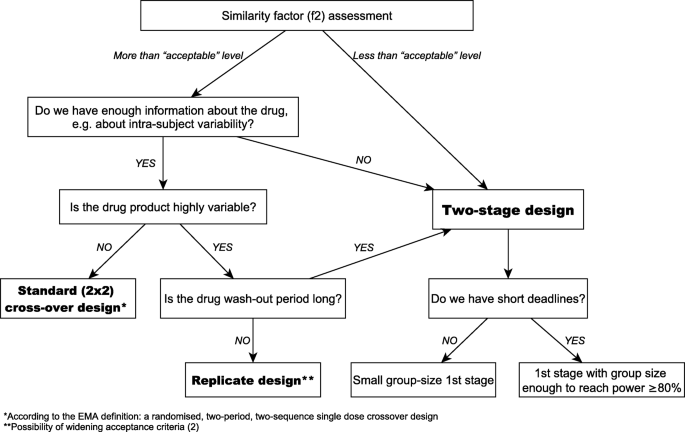
10th Anniversary of a Two-Stage Design in Bioequivalence. Why Has it Still Not Been Implemented? | SpringerLink

Framework of the EMA for average bioequivalence with expanding limits. | Download Scientific Diagram

In vitro and In silico biopharmaceutic regulatory guidelines for generic bioequivalence for oral products: Comparison among various regulatory agencies - Kollipara - 2021 - Biopharmaceutics & Drug Disposition - Wiley Online Library

Table 2 from Study of regulatory requirements for the conduct of bioequivalence studies in US, Europe, Canada, India, ASEAN and SADC countries: Impact on generic drug substitution. | Semantic Scholar

Bioavailability and bioequivalence studies in Turkey: A status report from the national registry of studies between 2008-2014 | Semantic Scholar

Overview of the European Medicines Agency's Development of Product-Specific Bioequivalence Guidelines. - Abstract - Europe PMC
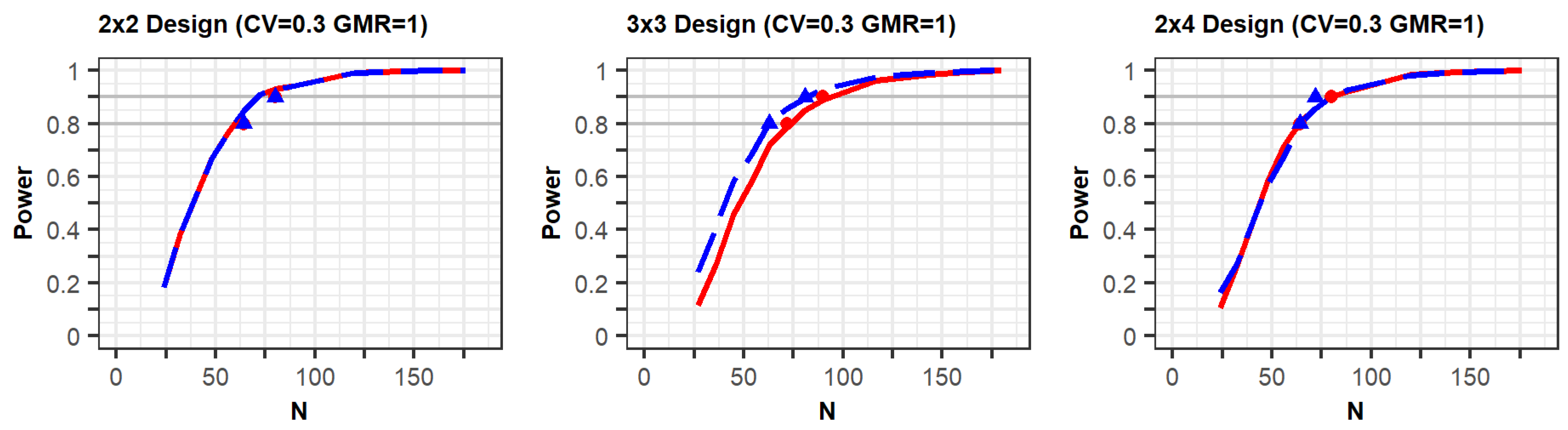
Pharmaceuticals | Free Full-Text | Model-Based Approach for Designing an Efficient Bioequivalence Study for Highly Variable Drugs
![PDF] The revised EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. | Semantic Scholar PDF] The revised EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/907abf8aeefcc48b34c28663f01713c6c394cbbb/6-Figure1-1.png)
PDF] The revised EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. | Semantic Scholar
Appendix IV of the Guideline on the Investigation on Bioequivalence (CPMP/EWP/QWP/1401/98 Rev.1): Presentation of Biopharmaceuti
Guideline on equivalence studies for the demonstration of therapeutic equivalence for locally applied, locally acting products i
![PDF] The revised EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. | Semantic Scholar PDF] The revised EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/907abf8aeefcc48b34c28663f01713c6c394cbbb/8-Figure2-1.png)
PDF] The revised EMA guideline for the investigation of bioequivalence for immediate release oral formulations with systemic action. | Semantic Scholar

News from the EMA: Guidelines announced on bioequivalence for immediate release solid oral dosage forms - GMDP Academy